500+ Cosmetic Product Recalls from European Market in 2024 H1
2024.09.30
In the first half of 2024, over 500 cosmetic products circulating in the European Union were identified as unsafe, prompting widespread product recalls. This trend highlights the critical need for vigilance in cosmetic safety and compliance.
The Safety Gate system, established across Europe, facilitates the rapid exchange of information regarding dangerous non-food products among national competent authorities. This system encompasses a wide range of consumer products, including:
- Chemical products
- Children’s products
- Electronic equipment
- Furniture
- Jewelry
- Motor vehicles
- Toys
- Cosmetics
Risks Associated with Recalled Cosmetic Products
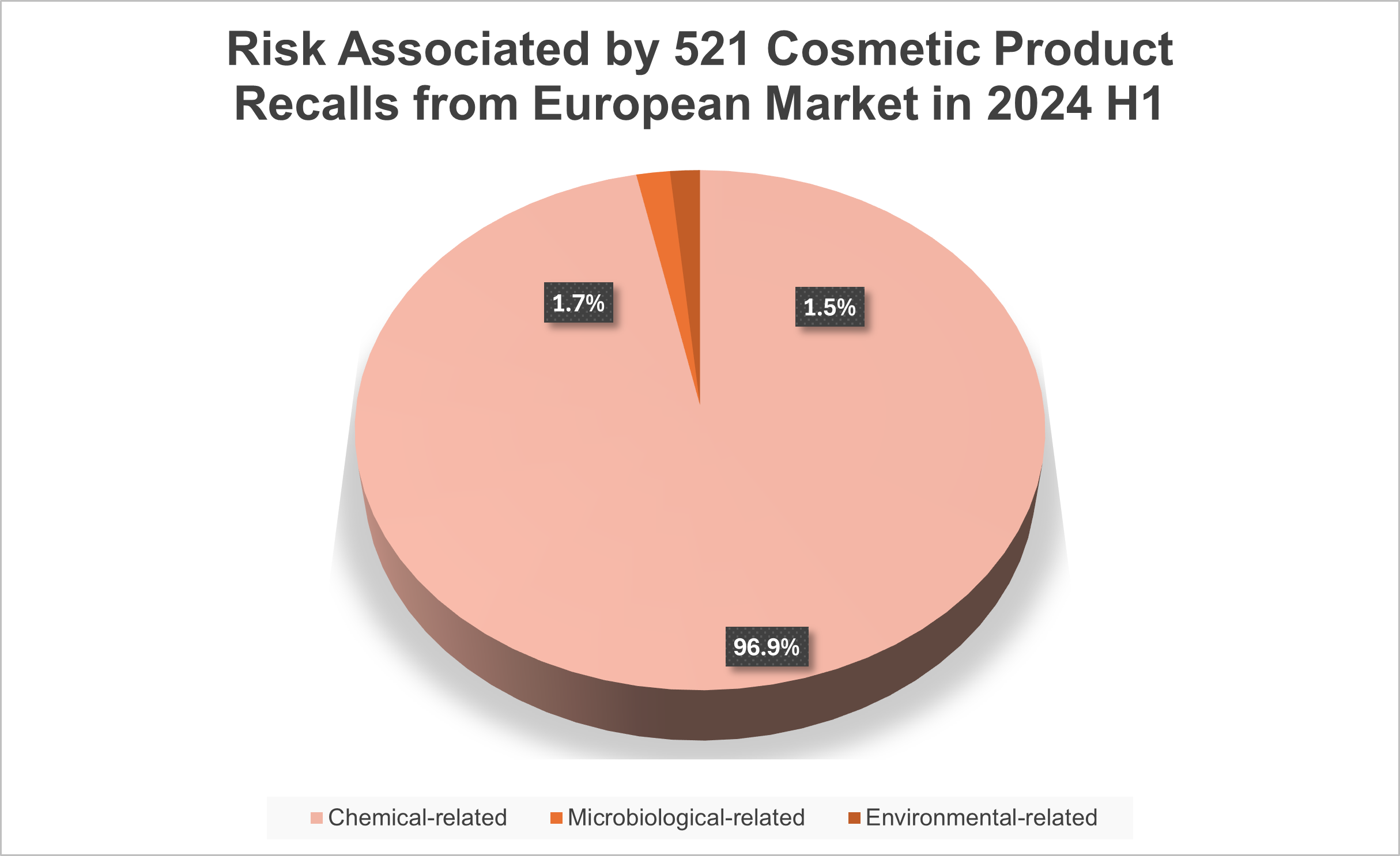
Among the recalled cosmetic products in the first half year of 2024, over 90% were associated with chemical risks, followed by less than 4% were linked to microbiological and environmental risks. Understanding these risks is crucial for ensuring consumer safety.
Key Substance Identifications
BMHCA
2-(4-tert-butylbenzyl) propionaldehyde (CAS No. 80-54-6), known as BMHCA, has been classified as a CMR (Repr. 1B) substance under Delegated Regulation (EU) 2020/1182. This substance has been prohibited from use in cosmetic products since March 1, 2022, as amended in Commission Regulation (EU) 2021/1902 of 29 October 2021.
Fragrance Allergens
Numbers of products were found to contain fragrance allergens above the labelling threshold, yet these substances were not listed in the ingredient declaration.
According to Cosmetic Regulation (EC) No. 1223/2009, the presence of substances, commonly called fragrance allergens, the mention of which is required under the column ‘Other’ in Annex III, shall be indicated in the list of ingredients. Fragrance allergens must be indicated when their concentration exceeds:
- 0.001% in leave-on products
- 0.01% in rinse-off products
One notable fragrance allergen, Hydroxyisohexyl 3-cyclohexene carboxaldehyde (HICC) (CAS No. 31906-04-4 / 51414-25-6), was detected in four recalled products. Due to its high potential for skin sensitization, HICC has been prohibited from use in cosmetics since August 23, 2021, as amended in Commission Regulation (EU) 2017/1410 of 2 August 2017.
Octamethylcyclotetrasiloxane (D4)
Several products, including eye gels and hair masks, were found to contain Octamethylcyclotetrasiloxane (D4) (CAS No. 556-67-2). D4 is classified as very persistent and bioaccumulative, posing risks to aquatic life and the environment, and might affect human fertility if inhaled or swallowed. Due to its hazardous properties, D4 is prohibited from use in cosmetic products from 12 June 2019, as amended in Commission Regulation (EU) 2019/831 of 22 May 2019.
Preservatives
Methylchloroisothiazolinone (MI) (CAS No. 2682-20-4) and the mixture of Methylchloroisothiazolinone (MCI) and Methylisothiazolinone (CAS No. 26172-55-4 / 2682-20-4 / 55965-84-9) were detected in leave-on products such as tanning products and skincare products. While these preservatives are allowed in rinse-off products at a maximum level of usage of 0.0015%. However, they are not allowed to use in leave-on cosmetic products.
Microbiological and Environmental Risks
Microbiological Risk
A few recalled cosmetic products were contaminated with harmful microorganisms, including Burkholderia cepacia, Klebsiella pneumoniae, as well as various molds and yeasts.
The Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation issued by The Scientific Committee on Consumer Safety (SCCS) and European Standard EN ISO 17516 ‘Cosmetics – Microbiology – Microbiological limits’ provides a set of hygiene limit, quantitatively and qualitatively, to finished cosmetic product.
Environmental Risk
Eight products, covering hair creams, hair serums, hair sprays, eyeliners, lip products, were found to contain perfluorononyl dimethicone, which is a perfluorooctanoic acid (PFOA) related compound. All recall alerts were submitted by Finland. PFOA, its salts and PFOA-related compounds are regulated in POPs Regulation, which aims to regulate against risks posed by organic substances which can accumulate in living organisms and damage their health. PFOA has been classified as a substance of very high concern due to its hazardous properties.
The identification of unsafe cosmetic products in the EU underscores the importance of stringent safety regulations and compliance in the cosmetics industry. As consumers become increasingly aware of product safety, manufacturers must prioritize transparency and adhere to regulatory standards to protect public health.
Please subscribe and contact us at TIC Mall for more details.











